The Vetoquinol Group, which has always been inventive, is permanently looking for innovations which are likely to meet it clients daily needs, whether they be vets, livestock farmers or pet owners.
Vetoquinol, where innovation is a team job
 At Vetoquinol, over 150 employees worldwide work to develop innovative products that answer our clients needs. Scientific Division, Research and Medical Division, Global Project Management Division… every Vetoquinol project is built in close collaboration by multidisciplinary teams and several departments. They work together to improve internal coherence and unlock synergies.
At Vetoquinol, over 150 employees worldwide work to develop innovative products that answer our clients needs. Scientific Division, Research and Medical Division, Global Project Management Division… every Vetoquinol project is built in close collaboration by multidisciplinary teams and several departments. They work together to improve internal coherence and unlock synergies.
Vetoquinol researchers explore the paths which lead from the active principle to the animal. A large amount of creative work is necessary in order to make treatment as efficient and easy to administer as possible, in other words, to make it useful and usable. In this process, teamwork is a key element of success when it comes to seeing projects through to their conclusion.
Pharmaceutical research, a long and complex process
 The development of a new veterinary drug requires time (on average between five and ten years), the participation of multidisciplinary teams and major investments of between €3 and €15 millions.
The development of a new veterinary drug requires time (on average between five and ten years), the participation of multidisciplinary teams and major investments of between €3 and €15 millions.
In the research process for veterinary drugs, galenic considerations are a key element: one must constantly invent pharmaceutical formulas which are easy to use for vets, livestock farmers and pet owners.
By always looking for the best dosage, the best moment, the best place to administer the drug, Vetoquinol acts both for the safety of the animal and its owner, and also for the profitability of the livestock farmer who will put his animals quickly back into production without any risk for the consumer.
Some examples where the galenic approach makes the difference:
- appetising pills, which are spontaneously taken by cats and dogs
- single dose injectable formulas with long-lasting effects, practical for injecting antibiotics in a herd.
- a dog treatment in liquid form, in a daily dose, easy for owners to accurately administer.
MARKETING AUTHORISATION, THE ULTIMATE OBJECTIVE OF PHARMACEUTICAL RESEARCH
The marketing of a drug for humans or animals hinges on receiving marketing authorisation (MA). Granted on the basis of an exhaustive file, MA proves that the product meets health authorities requirements in terms of quality, safety and efficacy. Once the product has been marketed, Vetoquinol continues to monitor the safety and efficacy of its drugs, using two observatories dedicated to drug monitoring and epidemiological surveillance.
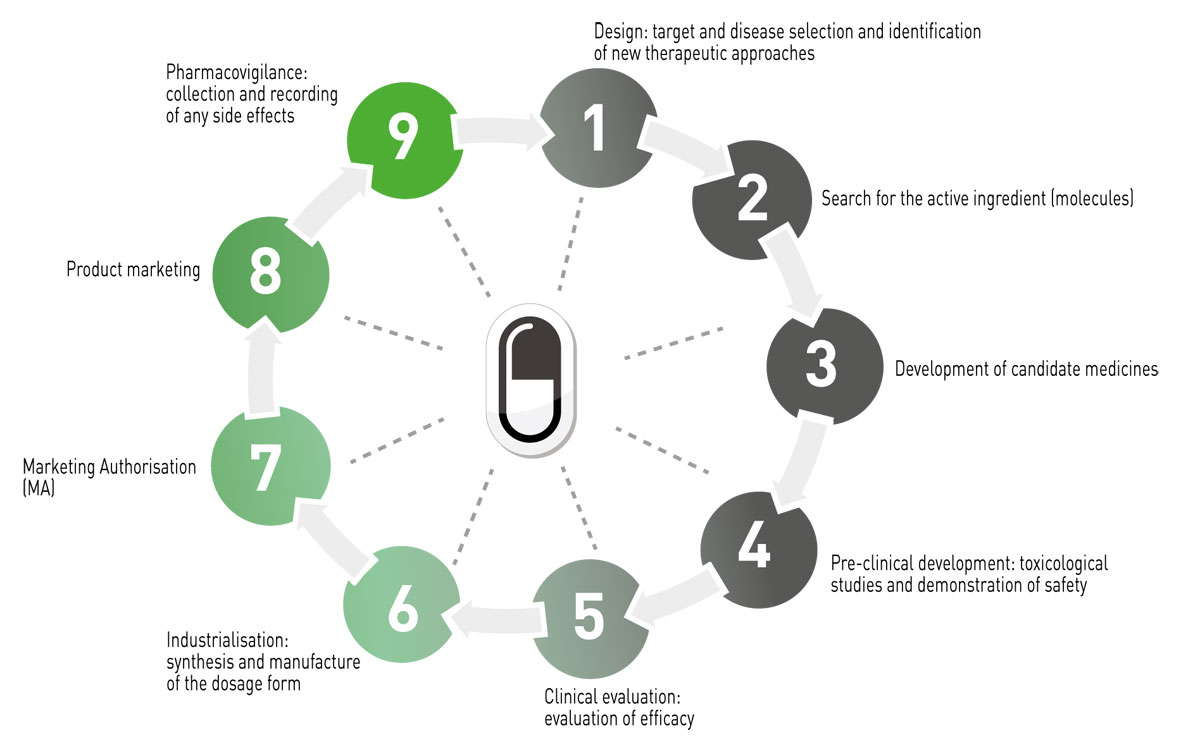
THE MAIN PHASES OF VETERINARY DRUG DEVELOPMENT